Breast Augmentation - Case 4
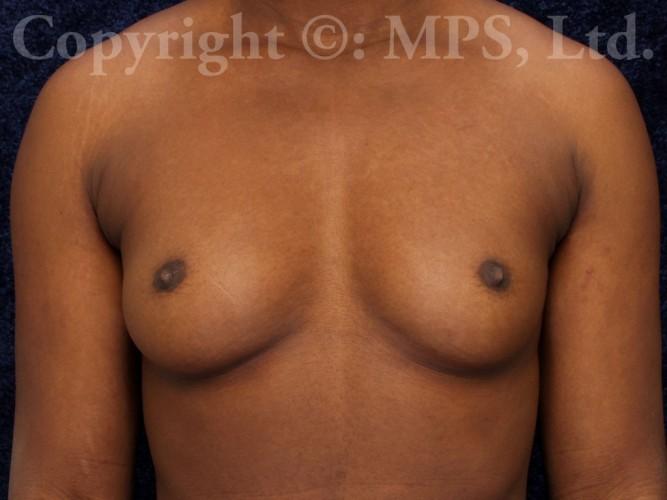 Before
Before
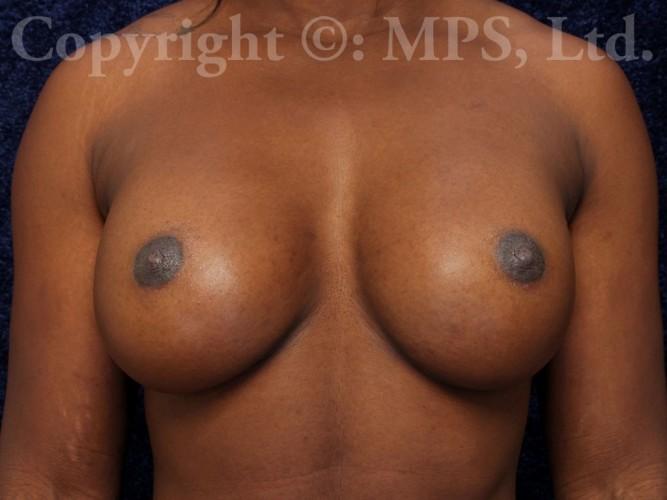 After (18 months post-op)
After (18 months post-op)
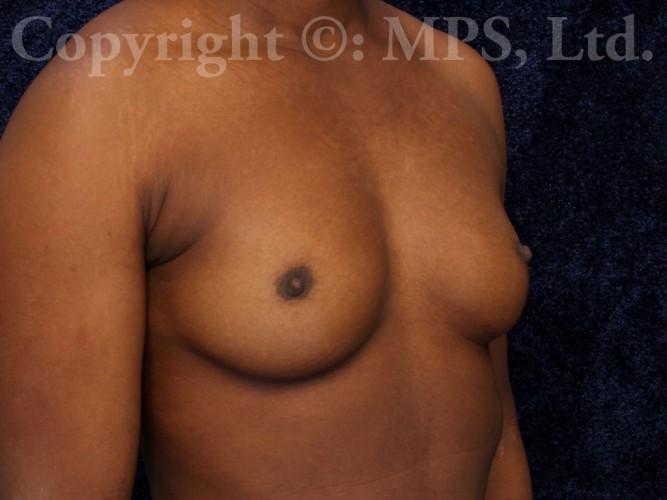 Before
Before
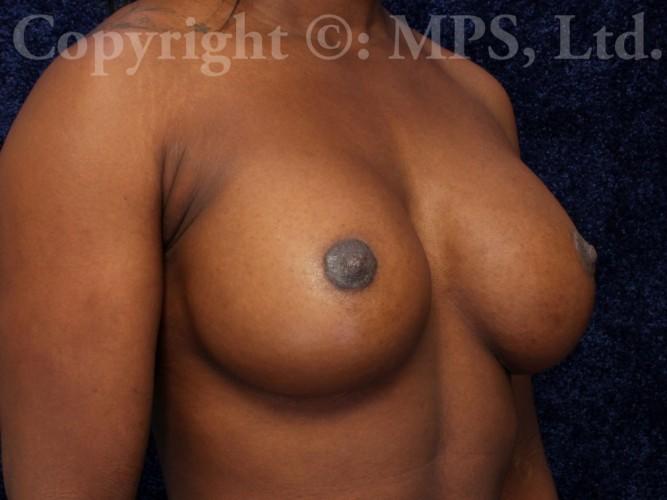 After (18 months post-op)
After (18 months post-op)
Case Description
This 20 year-old health care professional from Rochester, Minnesota wanted larger breasts. After much research, she decided she wanted the latest generation of cohesive silicone gel implants instead of saline ones. Even though she is not yet 22, it is perfectly legal and appropriate for her to request silicone gel implants, particularly when we agree that this is the best choice for her goals and anatomy. Use of silicone gel implants in a woman under age 22 is considered “off-label” use, just as when Botox is used for treatment of facial lines or wrinkles of the neck or upper forehead (Botox Cosmetic is FDA-approved only for use in treating frown lines between the brows and “crows feet” lines; use anywhere else is considered “off-label” use that is completely legal and quite common). She sized with 550cc and 600cc implants in a bra and stretchy top; in her 55 minute outpatient breast augmentation operation at Minneapolis Plastic Surgery, she had 650cc high-profile cohesive silicone gel implants placed. She has been thrilled with her results, and at her most recent office recheck appointment said that she is contemplating increasing to 800cc implants.
